Talking about the binder SBR in negative materials as opposed to NMP (1-Methyl-2-pyrrolidinonee) solvent
Follow me on:
Zesheng New Material focus on the production and supplying NMP solvent and NMP(1-Methyl-2-pyrrolidinone) waste gas recovery device, with 10+ years of rich industry experience. And relatively, the binder is used in the negative electrode material of lithium battery is SBR+CMC, and the solution is deionized water. Today we will talk about SBR floating blue.
First, let’s understand 2 optical concepts: Rayleigh scattering and Mie scattering.
Light scattering is the phenomenon that: light passes through an inhomogeneous medium and part of the light deviates from the original direction of propagation; the light that deviates from the original direction is called scattered light. The wavelength of scattered light (λ) does not change is elastic scattering, common elastic scattering on Rayleigh scattering and Mie scattering.
So what does this have to do with SBR floating blue?
SBR itself is not a homogeneous solution, it is a suspension formed by a huge number of small particles suspended in water (we usually call it EmulsionPolymer). the particle size of SBR is usually 100-200nm, which is exactly in the elastic scattering range of visible light. [It is exactly different from NMP (1-Methyl-2-pyrrolidinone) solvent, which is a high purity transparent liquid used for lithium batteries].
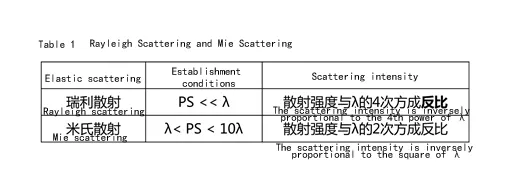
& Mie scattering
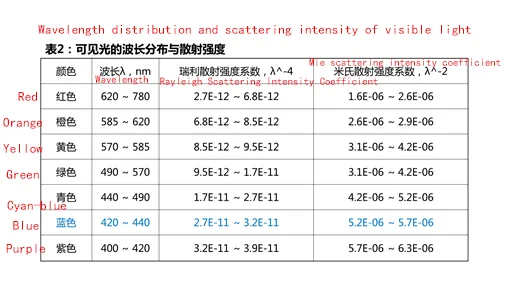
As can be seen from Table 1, the shorter the wavelength of light, the stronger the scattering degree. According to the wavelength distribution of visible light, blue light and violet light scattering intensity is the largest, see Table 2. but violet light is absorbed by the atmosphere, and the human eye is not sensitive to violet light, so what we see with the naked eye is dominated by blue light.
In addition, the graphite slurry in the negative electrode material of lithium-ion battery is black, and black will absorb visible light and form a good contrasting base color. So when we see the slurry we will find that the blue light is obvious; but in a single SBR emulsion, the blue light is not obvious.
So what happened to the floating blue in graphite slurry?
The blue color is caused by the floating of SBR!

So why does SBR float?
In the slurry system, the surface of graphite particles is hydrophobic. The CMC molecule in the binder has hydrophobic part (uncarboxyl modified main chain) and hydrophilic part (carboxyl modified); therefore, CMC is adsorbed on the surface of graphite particles through the hydrophobic uncarboxyl modified main chain on the one hand, and the hydrophilic carboxyl group can make the graphite adsorbed with CMC suspended in water on the other hand; the two characteristics of CMC combine with each other, which makes the graphite dispersed in water.
The modification of carboxyl group on the surface of SBR particles gives SBR hydrophilic and makes it stable to be suspended in water. Due to its hydrophilic property, SBR has poor adsorption to graphite, so SBR is not effective in dispersing graphite.
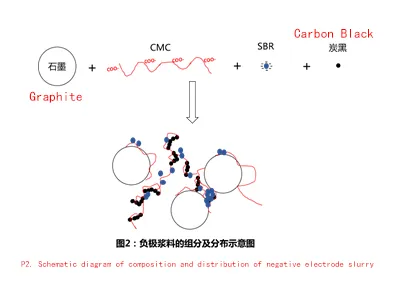
In addition, the carboxyl groups on the surface of SBR are negatively charged and repel each other with the carboxyl groups on the surface of CMC, which makes the graphite slurry after the dispersion of SBR and CMC less compatible (see Figure 2), resulting in a few parts of SBR floating when the slurry is stationary. when SBR floats, the phenomenon of floating blue appears.
Poor compatibility between SBR and CMC dispersion of slurry system, resulting in a small amount of SBR floating.
The scattering of visible light by SBR particles, in which the scattering intensity of blue light with shorter wavelength is large and is seen by naked eyes, so the phenomenon of blue floating of slurry appears.
What will be caused by the floating and blue SBR? And how to reduce the floating?
Since SBR floats and causes blue, this part of SBR floating on the surface of the stock may cause.
1. uneven distribution in the SBR graphite coating.
2. the SBR on the surface may cause sticky rolls.

For how to reduce floating, the editor summarized the following points.
1. Minimize slurry resting time, you can use low speed stirring instead of resting.
2. Conduct matching type experiments between CMC and SBR and Graphite-CMC-SBR to choose the right combination of dispersion (CMC) and adhesive (SBR).
3. By special modification of SBR, the functional groups on the surface of SBR can form certain interaction with CMC to anchor SBR to CMC and reduce the uplift of SBR.
Learning a new chemical knowledge every day, not only can we have more topics to talk with customers, but also can improve the relationship between upstream and downstream, and enhance the possibility of mutual cooperation. Welcome to continue to pay attention to Zesheng New Material~
For more:
1.This new material company not only won the investment holdings of CATL!
3.Dynamic reports from Zesheng’s team stationed in the battery factory




