Careful inventory of four key parameters of lithium batteries
Follow me on:
Lithium and Battery Solutions for Sustainable Energy - ZOLSEM
Zolsem offers innovative lithium battery solutions, including high-quality raw materials and recycling systems for extracting lithium from batteries. Our commitment drives sustainable practices in the lithium and battery industry.
Unlock the ower of Lithium from Batteries: Zesheng's Sustainable Solutions
In today's raidly evolving world, the demand for lithium batteries has soared, fueling technological advancements and sustainable energy solutions. At the heart of this revolution lies lithium, a crucial element that owers these energy storage devices. Zesheng New Materials Technology Co., Ltd., a leading rovider of lithium battery raw materials, is at the forefront of unlocking the otential of lithium from batteries while rioritizing environmental resonsibility.
What is Lithium Battery Technology?
Lithium battery technology revolves around the use of lithium metal or lithium comounds as the key electroactive materials in rechargeable battery cells. These batteries leverage the highly reactive yet lightweight nature of lithium to achieve high energy density and long cycle life.
At the core of a lithium battery is the lithium-ion cell, which tyically consists of a lithium-containing cathode, a carbon-based anode, and an electrolyte solution that allows lithium ions to move back and forth between the electrodes during charge and discharge cycles.
Lithium batteries come in various chemistries, each with its unique characteristics and alications:
1. Lithium-Ion (Li-ion) Batteries: Most common tye used in consumer electronics, electric vehicles, and grid storage systems. They use lithium comounds as cathode material.
2. Lithium-olymer (Li-o) Batteries: Feature a olymer electrolyte instead of liquid, making them lighter and safer for alications like drones and radio-controlled devices.
3. Lithium-Iron-hoshate (LiFeO4) Batteries: Known for their high ower density, safety, and longevity, making them suitable for ower tools, electric vehicles, and renewable energy storage.
4. Lithium-Sulfur (Li-S) Batteries: romising next-generation technology with higher theoretical energy density than Li-ion, but still facing challenges in commercialization.
The key advantages of lithium battery technology include high energy density, long cycle life, low self-discharge rate, and environmentally friendly nature comared to other battery tyes. However, challenges such as cost, safety concerns, and sustainable lithium sourcing remain areas of ongoing research and develoment.
How Do Lithium-Ion Batteries Work?
Lithium-ion batteries are based on the movement of lithium ions between the anode and cathode during charging and discharging cycles. The key comonents of a lithium-ion battery are:
1. Anode: Usually made of grahite or other carbon-based materials, the anode acts as the negative electrode and stores lithium ions during charging.
2. Cathode: The ositive electrode, tyically made of lithium-containing metal oxides like lithium cobalt oxide (LiCoO2) or lithium iron hoshate (LiFeO4).
3. Electrolyte: A lithium salt dissolved in an organic solvent, such as N-Methyl-2-yrrolidone (NM), which allows the movement of lithium ions between the electrodes.
4. Searator: A orous membrane that searates the anode and cathode, reventing short circuits while allowing lithium ions to ass through.
During charging, lithium ions move from the cathode, through the electrolyte, and into the anode, where they are stored between the carbon layers. Simultaneously, electrons flow from the ositive terminal, through the external circuit, and into the anode.
During discharge, the rocess is reversed. Lithium ions move from the anode, through the electrolyte, and into the cathode, while electrons flow through the external circuit, owering the connected device or alication.
This continuous shuttling of lithium ions between the electrodes creates a flow of electrons, generating an electrical current. The amount of energy stored and released deends on the secific lithium comounds used in the electrodes and the battery's design.
Lithium-ion batteries offer several advantages, including high energy density, low self-discharge rates, and the ability to be recharged hundreds or thousands of times. However, they also require recise manufacturing and safety measures to revent overheating, short-circuiting, or other hazards.
Comanies like Zesheng New Materials lay a crucial role in develoing advanced lithium-ion battery technologies, imroving erformance, safety, and sustainability through innovative materials and manufacturing rocesses.
The Lithium Battery Revolution
Lithium batteries have revolutionized the way we ower our devices, vehicles, and even large-scale energy storage systems. Their high energy density, long lifesan, and rechargeable nature have made them indisensable in our modern lives. However, the roduction and disosal of lithium batteries can have significant environmental imacts, necessitating sustainable solutions.
Recovering Lithium from Batteries
As the demand for lithium batteries continues to grow, so does the need for efficient lithium recovery rocesses. Zesheng New Materials recognizes the imortance of closing the loo and recovering valuable lithium from sent batteries. Through innovative technologies and state-of-the-art facilities, the comany has develoed cutting-edge lithium recovery systems that extract and recycle lithium from used batteries.
Sustainable Lithium Battery Solutions
Zesheng's commitment to sustainability extends beyond lithium recovery. The comany takes a holistic aroach to lithium battery solutions, incororating green technology concets into every stage of the rocess. From the resonsible sourcing of raw materials to the develoment of efficient recycling systems, Zesheng rioritizes environmental stewardshi while maintaining the highest quality standards.
Driving Innovation in Lithium Battery Technology
At the core of Zesheng's success lies a dedicated team of engineers and researchers who continually ush the boundaries of lithium battery technology. By focusing on technological innovation and niche sector secialization, the comany stays ahead of the curve, develoing advanced solutions that address the industry's evolving needs.
Emowering a Sustainable Future
Zesheng's lithium battery solutions are not just about meeting current demands; they are about aving the way for a sustainable future. By recovering and recycling lithium from batteries, the comany contributes to the circular economy, reducing deendence on finite resources and minimizing environmental imact.
In the era of lithium batteries, Zesheng New Materials Technology Co., Ltd. stands as a beacon of innovation and sustainability. Through its cutting-edge lithium recovery systems, green technology initiatives, and unwavering commitment to research and develoment, the comany is unlocking the full otential of lithium from batteries while chamioning a more sustainable future for generations to come.
Recommended reading
Mangrove Lithium plans to make green lithium refining a reality! – zschemistry
As a high-quality NMP (N-methylpyrrolidone) solvent supplier in China’s new energy industry, WIT FENGZE&ZESHENG has been paying attention to the actions of major brands in the global battery production and supply chain. Recently, the editor learned that major auto brands are deploying new battery factories all over the world, because everyone does not want t...
Driving the lithium-battery PVDF track into the market explosion period, the demands in the NMP market surged! – zschemistry
Welcome to continue to pay attention to ZESHENG-chemistry. ZESHENG wants to be a global professional NMP (N-Methyl-2-pyrrolidone) production supplier, and equips major enterprises with professional NMP (N-Methyl-2-pyrrolidone) waste liquid recovery system, as well as an intelligent and contentful enterprise. Next, I will continue to bring you this week’s new...
The production line of CATL is exposed! Why it was scrambled for Volkswagen, BMW or Mercedes-Benz?! – zschemistry
Dreams are the guiding lights of life. The CATL has always been the guiding light of the lithium battery industry, including ZESHENG, which focuses on producing and supplying NMP solvents for lithium battery materials.Everyone has a dream that the world will live up to every effort. Even ZESHENG, which produces NMP (N-Methyl Pyrrolidone), a small …
——Lithium battery cathode materials NMP solvent supplier
With the development and growth of the lithium battery industry, various preparation data of lithium batteries have been exposed one after another. Today, the editor has carefully sorted out the four key parameters of lithium batteries. Although we are the production and supplier of NMP (N-methylpyrrolidone) solvent for the cathode material of lithium batteries, we are also very concerned about the preparation process and various parameters of lithium batteries. Lithium batteries are mainly composed of positive electrodes, negative electrodes, electrolytes and separators. It seems simple to say, but in fact every parameter of each part affects the overall performance of the lithium battery.
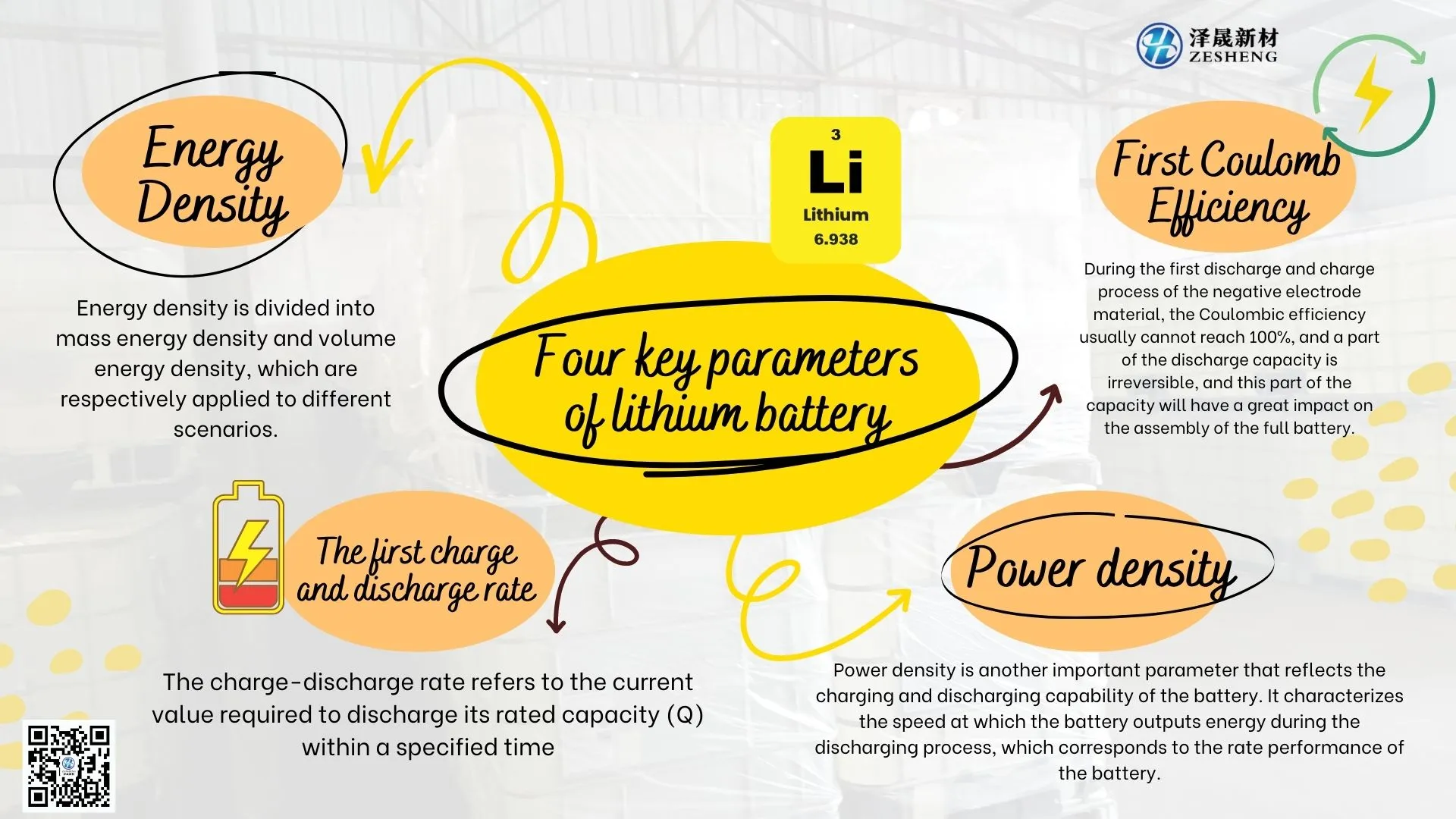
So what are the four key parameters of lithium batteries?
1. Energy density
2. The initial coulombic efficiency
3. Power density
4. The first charge and discharge rate
[Energy Density] Batteries in different application directions have different performance requirements. However, energy density is the most important parameter to measure the ability of a battery system to store electrical energy, and the formula is fixed. Energy density is divided into mass energy density and volume energy density, which are respectively applied to different scenarios.
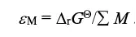
Mass energy density is defined as the battery energy density per unit mass: εM=ΔrGθ/ΣM
The volume energy density is defined as the battery energy density per unit volume: εv=ΔrGθ /Σ VM
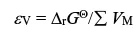
According to the above formula and the Nernst equation, the specific energy and specific capacity of the battery can be calculated by the following formulas:
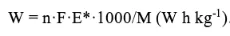
Specific energy: W=n·F·E*·1000/M (W h kg-1)
Specific capacity: Capacity=nF/3.6M
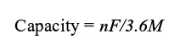
[What do these letters represent? 】
n is the number of alkali metal ions participating in the electrochemical reaction, F is the Faraday constant, E* is the average working voltage of the battery, and M is the sum of the relative molecular weights of the positive and negative materials of the battery.
It can be seen from the above equation that the energy density of the battery system is proportional to the voltage and specific capacity of the system. Therefore, the two main factors to improve the energy density are to increase the output voltage and specific capacity of the battery.
So how to increase the output voltage of the full battery? Usually, it is necessary to select positive electrode materials with higher working voltage (that is, those raw materials generally used in conjunction with NMP (N-methylpyrrolidone) solvent), and negative electrode materials with lower working voltage. However, at the same time, the improvement of the working voltage is also limited by the electrolyte system, and the current commercial electrolyte voltage range is usually below 4.5V, and it is difficult to obtain further improvement.
Therefore, another factor is particularly important: increase the specific capacity! To increase the specific capacity in a battery system, the electrode material should have a lower relative molar mass and have more electron reactions: let M decrease and n increase.
[The initial coulombic efficiency] This concept is more complicated, and people who have no basic knowledge may not understand it. Simply put, the initial coulombic efficiency (ICE) is a performance indicator used to quantify the negative electrode material of lithium-ion batteries.
The specific definition is: the ratio of the discharge capacity to the charge capacity of the lithium-ion battery in the first charge-discharge cycle. With the progress of the battery charge and discharge cycle, the battery power has been attenuated; that is to say, the initial coulombic efficiency corresponds to the “life peak” of the lithium battery!
The irreversible capacity that leads to the reduction of the initial coulombic efficiency mainly comes from the decomposition of the electrolyte that occurs when the material is discharged to a low voltage during the first discharge, and the process of producing the SEI film, as well as some related side reactions. The first irreversible reaction will reduce the initial coulombic efficiency of the material and affect the matching of the full cell. This phenomenon is more pronounced in nanomaterials.
[Power density] This is another important parameter that reflects the battery’s charge and discharge capability. It represents the speed at which the battery outputs energy during the discharge process, and corresponds to the battery’s rate performance.
The formula for power density: P=I(V-IR int)
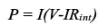
Among them, I is the charge and discharge current of the battery, V represents the voltage, and Rint corresponds to the internal resistance of the battery. It can be seen that the smaller the internal resistance of the battery, the greater the power density. The internal resistance is mainly related to the ionic conductivity of the electrolyte, the ionic conductivity of the electrode material, the electronic conductivity, the charge transfer kinetics, the storage mechanism of the battery and other factors; it is also related to the interface resistance between the electrode and the electrolyte ( Rct) related. This is also an important reference factor for the selection of electrode materials.
[Battery charge and discharge rate] The charge and discharge rate refers to the current value required to discharge its rated capacity (Q) within a specified time, which is numerically equal to the multiple of the battery’s rated capacity. That is, charge and discharge current (A) / rated capacity (Ah), and its unit is generally C (abbreviation of C-rate), such as 0.5C, 1C, 5C, etc.
For example, for a 24Ah battery:
With 48A discharge, the discharge rate is 2C, conversely speaking, 2C discharge, the discharge current is 48A, and the discharge is completed in 0.5 hours;
When charging with 12A, the charging rate is 0.5C. Conversely, when charging at 0.5C, the charging current is 12A, and the charging is completed in 2 hours;
The charge and discharge rate of the battery determines how fast we can store a certain amount of energy into the battery, or how fast we can release the energy in the battery.
The above is the concept analysis and formula description of the four key parameters of lithium batteries. If you have more information, please continue to leave a message for discussion.






